This page was last updated on 09-Apr-15
Revision exercises - try your hand at these.
These are not for a grade, just for practice. I shall be happy to help you if I can see you have tried first.
Alcohols revision - password required for copyright reasons
Alkanes revision - password required for copyright reasons
Reading exercises for 28 March 2015
Reaction of alchols with sodium
Replacing the hydroxyl group of alchohols with halogen
Formation of esters from alcohols
Aromatic compounds
Nitration of benzene and methyl benzene
Halogenation of benzene - for some reason they don't mention reaction with iodine here.
Acylation and alkylation of benzene
Sulfonation and other reactions of benzene and its derivatives
Reactions of benzene and its derivatives, with mechanism - this is all you need to know, and more.
Organic reaction mechanisms - select the ones you want.
The above list is quite a lot. Concentrate on what we cover in class.
Reading exercises for 23 March 2015
Haloalkanes - introduction - chemguide.co.uk previously recommended.
Mechanisms of reaction of organic halides - more advanced (previously posted, but revised 21 March 2015). Deals with the main mechanisms of reaction: SN1, SN2, E1, E2.
Reaction with hydroxide ions - chemguide.co.uk
Reaction with cyanide ion- chemguide.co.uk
Reaction with ammonia - chemguide.co.uk
Substitution reactions of haloalkanes - tutorvista.com - NB. Halide ions are not generally considered weak nucleophiles, contrary to what is mentioned on the linked page.
Most of this material is relevant to the course, but do not bother with the reactions involving "hydrosulphide" (HS-), nor "mecaptide" (RS-), nor "alkynyl" (-CɼC-R) (the last 3 reactions mentioned).
Reading exercise 15 March 2015
Plane polarised light and optically active compounds
Optical isomerism from Chemguide - make sure you try the questions
Optical isomerism from ChemWiki
Racemic mixtures and optical excess from ChemWiki
Meso compounds - master organic chem - This article mentions some different ways of representing the stereochemistry of a molecule, which students do not have to bother with. See below.
Enantiomeric excess and optical purity
Diastereoisomers and enantiomers - Sparknotes.com
Some of the above sources use sawhorse projections, e.g.
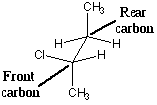
(Diagram from http://www.sparknotes.com/chemistry/organic3/enantiomersanddiastereomers/section2.rhtml)
According to the convention, the front carbon is always the lower left one. Not everyone seems to follow that system.
Another way of showing stereochemistry is the Newman projection, e.g.
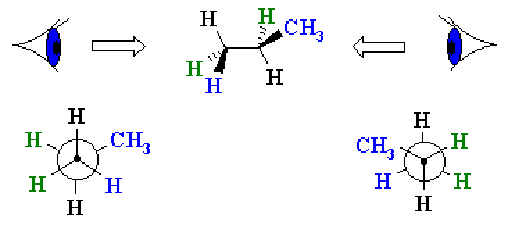
(Diagram from http://www.mhhe.com/physsci/chemistry/carey5e/Ch03/ch3-diagrams.html)
Here the front carbon is indicated by the dot, and the rear one by the circle.
Midterm
Alkanes
Alkenes
All previous material has been concentrated in the page linked above.
Monday 2nd March lecture: oxidation and polymerisation of alkenes.
Review this page for the oxidation of alkenes with cold, dilute, alkaline potassium permanganate (KMnO4) solution (a crude test for unsaturation) and hot KMnO4(aq) - a much stronger oxidising agent. (Details of the reactions are not required.) Another reaction we shall mention is "reductive ozonolysis". Also review this page about polymerisation of akenes. (This goes into more detail than required.)
Your marks so far
Chem230\Marks\C230_semester_01-2015(01).pdf
Homeworks for submission
Chem_230_H2 structures&isomers_01-20015.pdf
Chem_230_H1 bonding_01-20015.pdf
Chem_230_H3 01-20015.pdf - Answer key
Chem 230 H4 Optical isomerism - Answer key
Chem_230_H5_- synthesis and mechanism
Get up to speed with organic nomenclature - do these quizzes:
Multiple choice quizz - alkanes
Write in the name quizz - alkanes
(All the above are from Doc Brown's chemistry site.)
Reading exercises on nomenclature
Make sure you study these:
How to name organic compounds (1) - part of a large chemistry website in the UK
Naming organic compounds (2) - further functional groups present (Naming of amines does not seem to be in accordance with the IUPAC standard.)
Naming organic compounds (3) - aromatic compounds (derivatives of benzene)
E/Z and cis-/trans- naming convention
Keep abreast of what we do in class.
Isomerism in organic chemistry
Isomerism in organic chemistry from Chem Guide
Types of isomerism succinctly described - more advanced overview
Reading exercise on properties of alkanes
https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/funcrx1.htm
Videos
Documents
First prac and rules.pdf - superceded by:
Chem230\Documents\Chem230 manual 2015 part 1-1.pdf (Please use this version.)
How to write an organic lab report.pdf
Links for first few lectures
Introduction to electronic structure and bonding
Useful Links
All about organic solvents - polar, non-polar, protic, aprotic
Chemsketch - a free organic structure drawing tool
Guide to ChemSketch - gets you started quickly and easily
Doc Brown's chemistry website - look at the "A" level revision notes and quizzes
Uniqueness of carbon - visionlearning.com
Curly arrows in organic chemistry - an organic chemistry website
How to name organic compounds - A Michigan State University website
How to name
organic compounds - part of a large chemistry website in the UK
"Master organic chemistry" website
Emolecules - a site to find the structure of any molecule you can put a name to!
Graphical representation of stereochemical configuration - IUPAC recommendations
Useful Materials
Free on-line organic chemistry textbooks
Two in particular you might like to look at:
http://www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/intro3.htm#strc8c
http://chemwiki.ucdavis.edu/Organic_Chemistry/Organic_Chemistry_With_a_Biological_Emphasis/
Also: