This page was last updated on 04-Mar-15
Material for lectures on 18th, 22nd & 25th February
Note that a mechanism for the hydrogenation of ethene on a catalyst surface was given in the lab schedule. It is taken from Catalytic hydrogenation of alkenes. It applies to all akenes. Study and understand it.
In the meantime, study this website, which contains most of the material of the scheduled lecture:
In addition to the addition reactions
of akenes and their mechanisms, there are two types of rearrangement of
carbonations that I plan/planned to deal with: the hydride shift (which they
don't mention) and the methyl (or methide) shift which they do. The
hydride shift (transfer of H- to the next carbon atom) accounts
for the fact that treatment of, for example, 3-methylbut-1-ene,
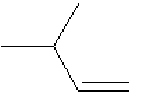 , with hydrogen
bromide, forms
, with hydrogen
bromide, forms
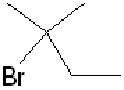 in greater amount than the expected
in greater amount than the expected
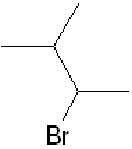 . See if
you can come up with the mechanism after reading about the reactions of alkenes.
Don't bother with the reaction of α-pinene, it's
unnecessarily complicated.
. See if
you can come up with the mechanism after reading about the reactions of alkenes.
Don't bother with the reaction of α-pinene, it's
unnecessarily complicated.
N.B. Carbocation rearrangements are not useful preparatively, since they don't normally give good yields. They are important by virtue of the fact that they often mess up reactions that we hope to carry out!
A term that will not be familiar to you is "α" (alpha) as in "α-pinene" or "α-halo-alcohol or ether". This refers to a carbon next to the carbon in a functional group. 2-chloroethanol is an α-halo-alcohol, since the chlorine is attached to the carbon atom next to that bearing the -OH group. Another term is "vic-dihalide". "vic" is short for "vicinal" (explained in the text) which means neighbouring. 1,2-dichloroethane is a vic-dihalide, since the chlorines are on neighbouring carbon atoms.
The Hammond postulate is explained better than I explained it in class.
The terms "regioselective" and "stereoselective" are introduced and explained. This material connects with what was done in the Friday practical.
We are not studying hydroboration nor oxymercuration. Suffice it so say that hydroboration results in the anti-Markovnikov addition of H-OH and oxymercuration gives the Markovnikov addition of H-OH, but with no complications from the rearrangement of carbocations.
Don't
bother with "epoxidation" (formation of "epoxides" such as
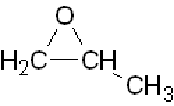 ).
).
If you like videos, here are some very good ones from http://leah4sci.com/:
-
Halogenation of alkenes NB resonance is misrepresented here. It does NOT happen "back and forth". There is only one form of the cyclic bromonium (or more generally, halonium ion) intermediate. Here's the one formed from propene:
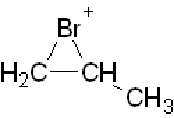
You may say there are three contributing structures, putting the positive charge on the bromine as shown above, as well as on each of the two neighbouring carbons, but these are not structures in their own right. In short, they do not exist, despite what the video implies! They just illustrate that the positive charge is distributed between the three atoms (with more on a secondary carbon than on a primary one).
-
Acid catalysed hydration of alkenes NB A pH of 1 does not mean 1 H+ for every 10 H2O molecules. It means 0.1 mol of H+ for every litre of water. A litre of water is about 55.6 mol, so that means 1 H+ for every 556 water molecules. And so on for pH2.
-
Hydride and alkyl shifts in carbocations (It ought to be an "alkide" (or methide) shift, since the species that moves carries both the bonding electrons with it, and therefore bears a negative formal charge.)
NB. Reactions that involve a more unstable intermediate happen more slowly than reactions which involve a more stable intermediate as a consequence of the "Hammond postulate". This is explained in Reactions of alkenes 1.
Previous material
-
Nomenclature of alkenes - basic
-
Nomenclature of alkenes - more detail and more examples
-
Structure, isomerism and nomenclature of alkenes - good basic stuff
-
Reactions of alkenes - Start by reading "background", "making alkenes", "hydrogenation", "reactions with halogens" (iodine in fact reacts only partially and reversibly), "reactions with hydrogen halides", "reaction with sulfuric acid". You will need to be familiar with most of the material given here. Also review the other links listed.
-
Reactions of alkenes - mechanisms of electrophilic addition reactions - read all the material here. You will need to be familiar with it.
-
Leah's organic chemistry site - reactions of alkenes (videos) (I haven't identified specific videos yet, but they seem very good.)